
|
Current Microbiology
Publisher: Springer New York
ISSN: 0343-8651 (Paper) 1432-0991 (Online)
DOI: 10.1007/BF01577723
Issue: Volume 8, Number 5
Date: September 1983
Pages: 251 - 253
|
Mutants of Escherichia coli sensitive to hydrogen peroxide Casimiro Barbado1, Manuel Ramírez2, M. Angel Blanco1, J. López-Barea1, 3
 and Carmen Pueyo2 and Carmen Pueyo2 | (1) | Departamento de Bioquímica, Fac. Ciencias, Univ. Extremadura, Badajoz, Spain |
| (2) | Departamento de Genética, Fac. Ciencias, Univ. Extremadura, Badajoz, Spain |
| (3) | Present address: Departamento de Bioquimica, Fac. Veterinaria, Univ. Córdoba, Cordoba, Spain |
Abstract Mutants sensitive to moderate H 2O 2 concentrations were selected in a Hfr phoA (S33) Escherichia coli strain after mutagenesis with N-methyl, N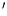 -nitro, N-nitrosoguanidine
(NG). Of the sensitive strains, 31% were catalase-deficient and
retained glutathione reductase levels similar to those of the parental
strain, whereas 69% still had normal catalase and glutathione reductase
activities. Mutants supersensitive to low H 2O 2
concentrations were selected in a catalase-deficient strain (CGR201)
after mutagenesis with NG. Of these, 20% were glutathione
reductase-deficient, and the remaining 80% were unaffected in this
enzymatic activity. Compared with the parental strain S33, H 2O 2 was 5 to 12 times more toxic for the sensitive mutants, and 19 to 21 times for the supersensitive ones.
The references of this article are secured to subscribers.
Referenced By:  This article is referenced by 1 newer article... This article is referenced by 1 newer article...
|
|
| |
|